Ensuring responsible antimicrobial use in EU food production
MEP Interest Group on AMR Annual Meeting
29 June 2021
15:00-17:30 CEST
Online
Join the MEP Interest Group for their annual meeting on 29 June to discuss opportunities for the transition to a sustainable model of food production that ensures responsible antimicrobial use and contributes to the global response to tackling AMR
Welcome by

Sarah Wiener, MEP
Chair of the MEP Interest Group on AMR
Greens/EFA, Austria

Susana Pombo
Director-General
Directorate-General of Food and Veterinary, Portugal
Session 1: Towards a sustainable food system in the EU
The Veterinary Medicinal Products and Medicated Feed Regulations will put in place a wide range of provisions to support responsible use of medicines, particularly antimicrobials, paving the way for a list of medically important antimicrobials reserved for human use as well as a system of data collection on antimicrobial use across species.
However, there are still concerns about the readiness of EU governments and the food production sector to implement and comply with the provisions or if they are sufficiently aware of the system changes needed to move towards a more sustainable model of food production.
- What are the barriers to more sustainable animal husbandry that minimises antimicrobial use? How can animal welfare be improved?
- What critically important antimicrobials should be reserved for human use?
- How can innovation and technology support implementation of best practices and minimise the need for antimicrobial use?
- How can we ensure that pharmaceuticals not classified as antimicrobials will not be used to compensate for poor animal husbandry?

Sirpa Pietikäinen MEP
Member, MEP Interest Group on AMR
EPP, Finland

Jeroen Dewulf
Professor in Veterinary Epidemiology
Ghent University

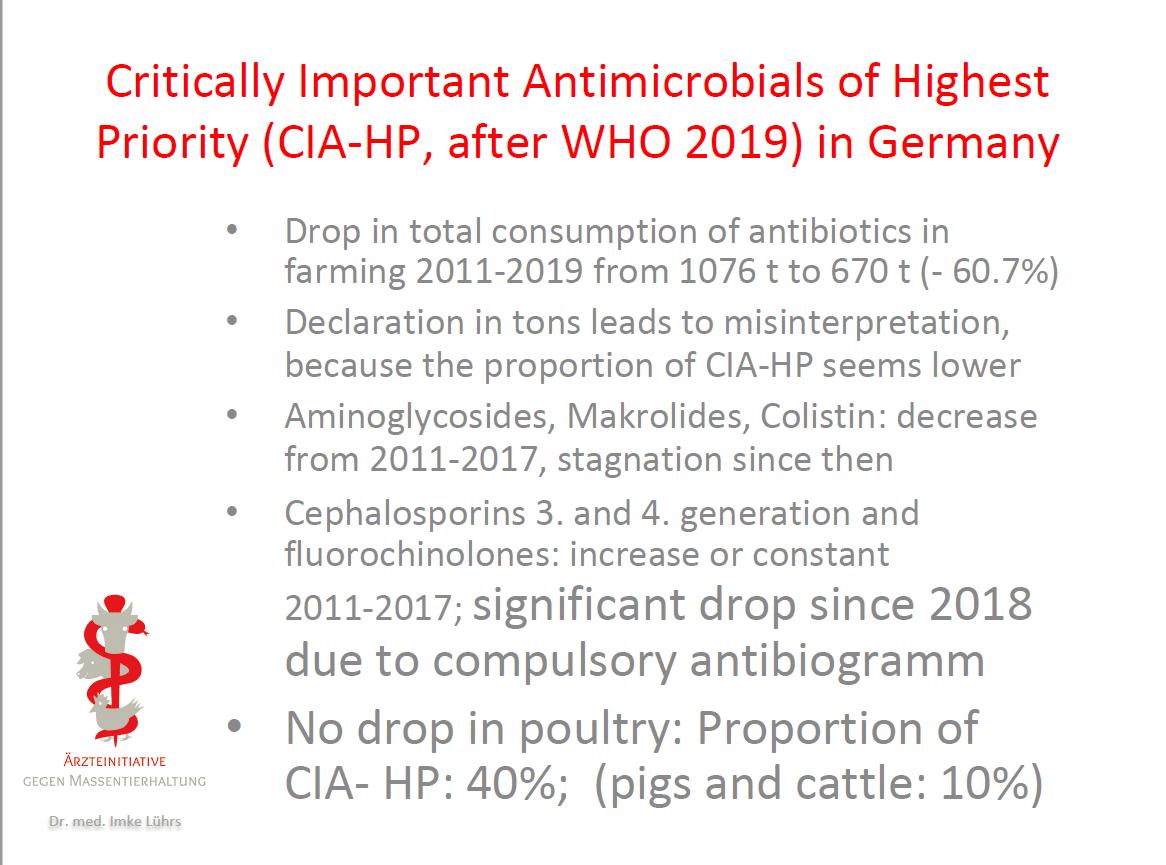
Imke Lührs
Board Member
Doctors Against Factory Farming

Peter Stevenson
Chief Policy Advisor
Compassion in World Farming

Eva Maria Zamora Escribano
Head of Unit for Animal Nutrition and Veterinary Medicines
European Commission
Download the Presentations
Session 2: Shaping the global agenda
Third-country operators in many parts of the world do not produce food to the high standards of the EU, yet they will not be subject to the EU ban on the prophylactic use of antimicrobials for groups of animals, posing a threat to European consumers and exposing EU food producers to unfair competition.
The EU has a key role to play in driving markets and advocating for changes at the global level such as concerning the use of antimicrobials as growth promoters. The global agreement on the use of antimicrobials and the chapter on sustainable food systems of free trade and investment agreements are additional potential avenues for the EU to shape the global agenda.
- How can we ensure a level playing field for food imports when prophylactic use of antimicrobials for groups of animals will still be allowed?
- What would the food production component of a global agreement on the use of and access to antimicrobials look like?
- How can innovation and technology support implementation of best practices and minimise the need for antimicrobial use?
- How can we ensure that pharmaceuticals not classified as antimicrobials will not be used to compensate for poor animal husbandry?

Manuela Ripa, MEP
Member, MEP Interest Group on AMR
Greens/EFA, Germany

Stephanie Ghislain
Trade and Animal Welfare Programme Leader
Eurogroup for Animals

Keith Sumption
Chief Veterinary Officer, Animal Production and Health Division
Food and Agriculture Organisation (FAO)

Birthe Steenberg
Secretary-General
AVEC EU Poultry

Koen Van Dyck
Head of Unit for Bilateral International Relations
European Commission
Get the EPHA Newsletter
The best of our activities, right in your inbox!